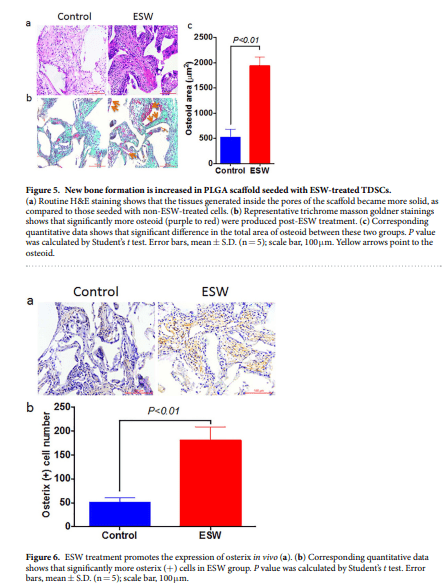
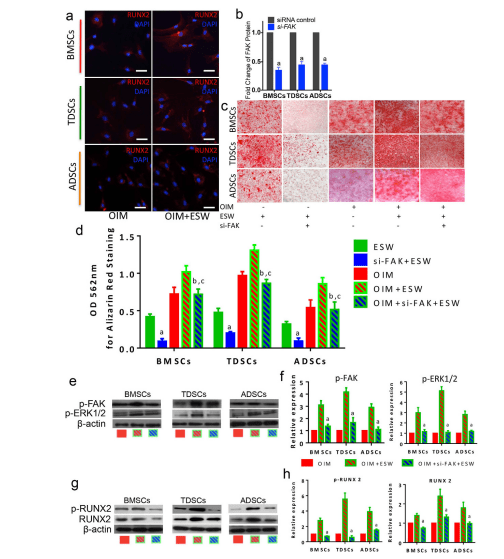
ESWT has been shown to have great potentional in promoting the osteogensis of bone marrow mesenchymal stem cells
Focal Adhesion Kinase Signaling
Mediated the Enhancement
of Osteogenesis of Human
Mesenchymal Stem Cells Induced
by Extracorporeal Shockwave
Extracorporeal shockwave (ESW) has been shown of great potential in promoting the osteogenesis
of bone marrow mesenchymal stem cells (BMSCs), but it is unknown whether this osteogenic
promotion effect can also be achieved in other MSCs (i.e., tendon-derived stem cells (TDSCs)
and adipose-derived stem cells (ADSCs)). In the current study, we aimed not only to compare the
osteogenic effects of BMSCs induced by ESW to those of TDSCs and ADSCs; but also to investigate
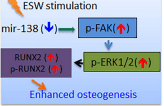
the underlying mechanisms. We show here that ESW (0.16mj/mm2) significantly promoted the
osteogenic differentiation in all the tested types of MSCs, accompanied with the downregulation of
miR-138, but the activation of FAK, ERK1/2, and RUNX2. The enhancement of osteogenesis in these
MSCs was consistently abolished when the cells were pretreated with one of the following conditions:
overexpression of miR-138, FAK knockdown using specific siRNA, and U0126, implying that all of these
elements are indispensable for mediating the effect of ESW. Moreover, our study provides converging
genetic and molecular evidence that the miR-138-FAK-ERK1/2-RUNX2 machinery can be generally
activated in ESW-preconditioned MSCs, suggesting that ESW may be a promising therapeutic strategy
for the enhancement of osteogenesis of MSCs, regardless of their origins.
Extracorporeal shockwave (ESW) has been widely used in musculoskeletal disorders such as bone defects
(delayed- and non-union of bone fractures, avascular necrosis of femoral head), with a success rate around 80%,
while the complications are low or negligible1
. ESW in orthopaedics is not used to disintegrate tissues, rather to
induce tissue repair and regeneration2
. Several ESW devices have been approved by the United States Food and
Drug Administration (FDA) as Class III orthopedic lithotripsy devices3